Clinical Trials
Written by: Dr Jared John, Dermatology Clinical Research Fellow, Sinclair Dermatology
What are clinical trials and why are they important?
Recent medical advances have improved our understanding of disease pathways that in turn has led to the development of targeted treatments that are more effective and have fewer side effects.
However, before the Therapeutic Goods Administration (TGA) approves any new drug for use and sale in Australia, it must pass many phases of rigorous assessments to ensure it is both safe and effective. This takes many years to complete and begins with preclinical research using computer simulation and animal testing before advancing to clinical trials in humans.
Clinical trials are research studies that recruit and assign participants to one or more health interventions to evaluate their health outcomes. Clinical trials are essential to the development of new treatments as they provide real-world information about how a drug works. They help determine the lowest effective dose, whether there are any safety concerns and how well they fix the problem they are intended for.
What are the different phases of clinical trials?
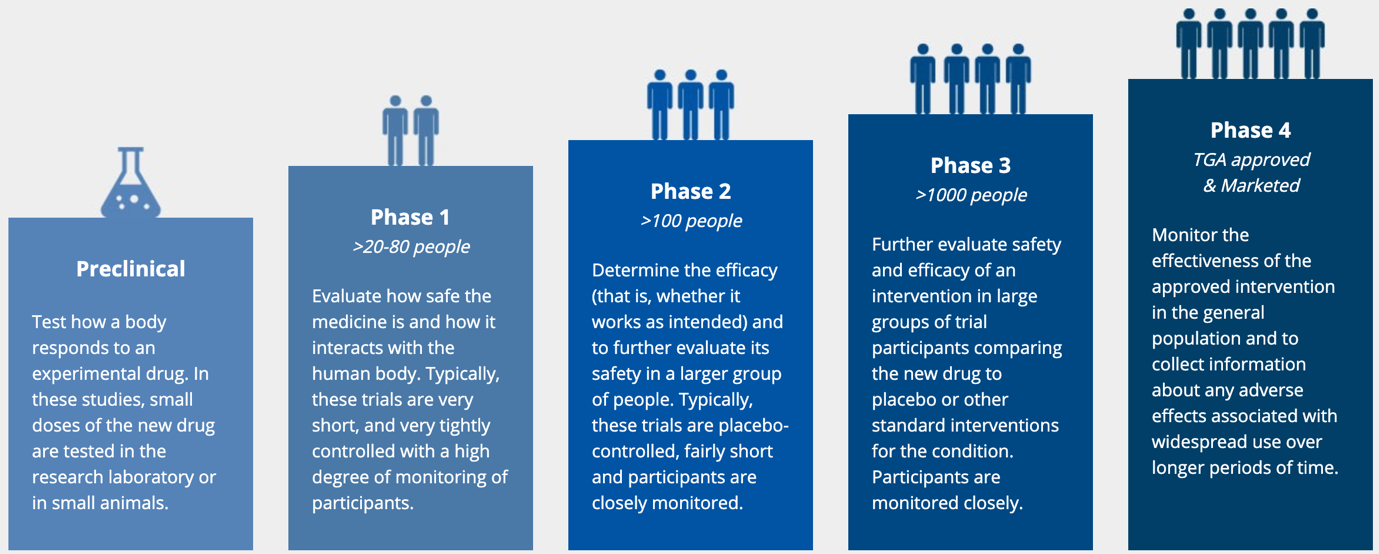
Clinical trials are typically conducted in 4 phases. Initially, the phase 1 study test the medication in a handful of participants to assess safety and effectiveness. If the drug works and there are no safety concerns the trial will progress to the next phase. In phase 2, dose ranging studies are performed to determine the optimum dose that balances effect and safety. In phase 3 the optimal dose is tested in thousands of volunteers so that the benefits can be accurately measured and quantified. Any safety issues flagged in phase 1 and 2 studies are carefully monitored. There is always a placebo group in phase 2 and 3 studies to ensure the benefits gained are due to the medication and not a manifestation of natural or seasonal fluctuations in the disease being treated. Similarly, the frequency of side effects is compared to the placebo group to see whether they occur more commonly in people who take the medication. This is especially important for side-effects such as headache, nausea or runny nose that can occur in the people not taking any medication. Most new potential drugs never make it through to stage 4.
Are clinical trials safe?
Clinical trials do involve some risk, and therefore before any trial can commence in Australia, there is a legal requirement that the trial protocol and all the supporting research and documentation must be thoroughly evaluated by a human research and ethics committee (HREC). This process is designed to safeguard participants. Formal written approval by HREC must be received before any clinical trial can commence. The HREC insist upon extensive laboratory safety testing before human clinical trials.
The National Health and Medical Research Council (NHMRC) is the Commonwealth Statutory body that regulates the safety and wellbeing of participants, in clinical trials conducted in Australia and accredits and oversees the HRECs
The NHMRC is Australia’s peak body for supporting health and medical research and provides advice on ethical behaviour when conducting medical research. The HREC operates within NHMRC guidelines and reviews the scientific validity of the trial design, the balance of risk to harm of the intervention, the ethical acceptability of the trial process and approves the trial protocol. It is also responsible for monitoring the conduct of the trial. The TGA evaluates the clinical trial once it has been completed and determines whether the data obtained are sufficient for the medication to be registered as an approved medication. The TGA can request additional studies prior to registration. Once registered the TGA regulates the use of that therapeutic goods and continue to monitor its’ safety for the life of that product.
The risks that relate to participation in any trial are detailed in the Participant Information and Consent Form (PICF).
This must be read and understood before a volunteer can provide informed consent. A member of the research team will explain the study in detail, including its risks and benefits as well as your rights and responsibilities. They will answer your questions and provide the PICF for you to read and sign before commencing any trial activity. Participation in a clinical trial is voluntary and you are free to withdraw at any time.
What should I expect from a clinical trial?
Clinical trials require a time commitment. They may run for weeks to months, depending on the trial phase and design. Appointments can be as frequent as weekly. There is a chance you may receive a placebo instead of the active medication if you participate in a placebo-controlled trial. Moreover, the new medication may not work for every participant. Details of the trial are outlined in the PICF and explained at the screening visit (first appointment). At the screening visit, the investigator will gather as much information as possible about you and conduct initial assessments to determine if you are eligible to participate in the trial and whether it is appropriate for you to participate. Some investigations may be ordered and you will be provided with the consent to read so that you are fully informed about the potential benefits, the risks and the time commitment involved if you decide to go ahead. If you are eligible to enter the trial and decide to proceed, you will be randomised to a treatment arm in the trial and begin receiving medication at your next visit (baseline). At this and every subsequent visit, you will be asked about your general health and whether you experienced any adverse events. You may be required to keep a dosage or event diary. You will also be examined and monitored closely through blood and urine tests.
What are the benefits of joining a clinical trial?
Clinical trials conducted today are high-quality validated studies that are well regulated and closely monitored. Some of their benefits include:
- Access to new medications that are not yet available to the public
- Treatment is provided free
- Treatment may be more effective with fewer or no side-effects
- You will be cared for by health professionals who understand your condition
- Your overall health will be closely monitored
- You will play a more active role in your health care
- You will have greater access to resources and support groups
- You will be contributing to the advancement of clinical research and drug development
- You will be compensated for you time
There has never been a more exciting time to get involved and be part of valuable clinical research. Sinclair Dermatology is Australia’s leading dermatological research centre and Victoria’s largest dermatology clinic. Anyone interested in enrolling or learning more is encouraged to reach out to us at (03) 9013 0099 or clinicaltrials@sinclairdermatology.com.au. Our friendly and experienced staff will be able to answer your trial-related questions and steer you towards an appropriate trial.
References / Resources
- https://www.sinclairdermatology.com.au/clinical-trials/
- https://www.nia.nih.gov/health/clinical-trials
- https://clinicalstudies.com.au/faq/
- https://medicinesaustralia.com.au/policy/clinical-trials/
- https://www.australianclinicaltrials.gov.au/consumers
- https://www.tga.gov.au/publication/australian-clinical-trial-handbook
- https://www.nhmrc.gov.au/applicable-laws-and-obligations




